Introduction:
Liquid-state NMR plays a pivotal role in resolving the structural mysteries of molecular compounds across diverse fields such as synthetic organic and inorganic chemistry, as well as medicinal chemistry.
The Role of Computers in Structural Problem Resolution:
The use of computers is not just helpful but often essential in resolving structural problems. Computer-based structural elucidation (CASE) such as Mnova Structure Elucidation proves invaluable, particularly in navigating challenges posed by molecules with a poor hydrogen to carbon ratio. Computational techniques become increasingly relevant when determining stereochemical configuration and conformation.
Steps in Computational Structural Elucidation:
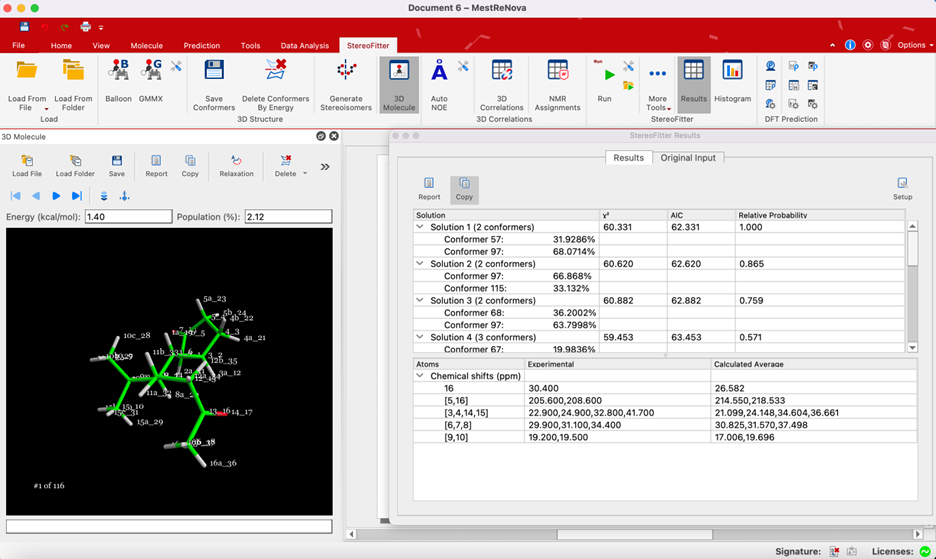
- Generation of Stereoisomers: Automation alleviates the tedium associated with generating diastereoisomers.
- Conformational Search: Molecular mechanics force-fields aid in exploring conformational space.
- Structural Refinement: Density functional theory (DFT) geometry optimization refines structural accuracy.
- NMR Properties Computation/Analysis: Various NMR observables contribute to structural analysis.
Employing Different NMR Observables:
Utilizing Different NMR Observables: The application of various NMR observables adds a subtle dimension to structural analysis, allowing for a comprehensive understanding of molecular characteristics. Each observable contributes unique insights, enhancing the precision and reliability of the elucidation process.
Beyond the Use of NOE Effects:
While NOE effects traditionally serve a qualitative role in structural analysis, their potential goes beyond. Rigorous analysis of kinetic NOESY/ROESY 1D or 2D experiments, following well-described protocols, yields accurate internuclear proton-proton distances. This data proves exceptionally effective in conformational analysis, particularly in complex medicinal chemistry problems. (1)
J-Couplings and Karplus-like Relationships:
Vicinal J-couplings provide essential dihedral angle information, crucial for stereochemical analysis. Empirical Karplus-like relationships allow quantitative use aspect of this information. Additionally, DFT predictions offer not only more accurate data but also the flexibility to incorporate various couplings, such as geminal 1J(C-H), 2J(C-H) couplings, or even the possibility to establish Karplus relationships based on DFT analysis of model systems.
Chemical Shift Analysis Supported by DFT Predictions:
Chemical shift analysis of 13C and 1H data, supported by DFT computations, has become increasingly popular for structural elucidation of natural products. This method proves valuable not only for de novo structural determination but also for the revision of already reported compounds.
Residual Dipolar Couplings (RDC):
RDCs emerge as a formidable tool for establishing configuration and conformation due to their capability to correlate the configuration of far away stereocenters. (2) They are applicable to a spectrum of structural problems, ranging from classical natural product elucidation, to medicinal chemistry applications such as the conformation guided design of cyclic peptides. (3) Weakly-aligning media, such as different types of liquid crystals or polymer gels, provides a user-friendly approach to the measurement of RDCs. (4)
In summary, the strategic incorporation of these diverse NMR observables enhances the analytical arsenal, enabling a thorough and efficient exploration of structural complexities. Mestrelab's commitment to providing tools like Mnova StereoFitter empowers users to seamlessly integrate and analyze this wealth of NMR and structural data for conclusive structural elucidation.
You can watch a demonstration of a related application in one of our webinars held in November 2023. "Advanced NMR Strategies for Pharmaceutical Molecule Characterization and Structure Analysis"
Bibliography:
(1) Thompson, A. A.; Harbut, M. B.; Kung, P.-P.; Karpowich, N. K.; Branson, J. D.; Grant, J. C.; Hagan, D.; Pascual, H. A.; Bai, G.; Zavareh, R. B.; Coate, H. R.; Collins, B. C.; Côte, M.; Gelin, C. F.; Damm-Ganamet, K. L.; Gholami, H.; Huff, A. R.; Limon, L.; Lumb, K. J.; Mak, P. A.; Nakafuku, K. M.; Price, E. V.; Shih, A. Y.; Tootoonchi, M.; Vellore, N. A.; Wang, J.; Wei, N.; Ziff, J.; Berger, S. B.; Edwards, J. P.; Gardet, A.; Sun, S.; Towne, J. E.; Venable, J. D.; Shi, Z.; Venkatesan, H.; Rives, M.-L.; Sharma, S.; Shireman, B. T.; Allen, S. J. Identification of Small-Molecule Protein–Protein Interaction Inhibitors for NKG2D. Proc. Natl. Acad. Sci. 2023, 120 (18), e2216342120. https://doi.org/10.1073/pnas.2216342120.
(2) Liu, Y.; Saurí, J.; Mevers, E.; Peczuh, M. W.; Hiemstra, H.; Clardy, J.; Martin, G. E.; Williamson, R. T. Unequivocal Determination of Complex Molecular Structures Using Anisotropic NMR Measurements. Science 2017, 356 (6333), eaam5349. https://doi.org/10.1126/science.aam5349.
(3) Farley, K. A.; Che, Y.; Navarro-Vázquez, A.; Limberakis, C.; Anderson, D.; Yan, J.; Shapiro, M.; Shanmugasundaram, V.; Gil, R. R. Cyclic Peptide Design Guided by Residual Dipolar Couplings, J-Couplings, and Intramolecular Hydrogen Bond Analysis. J. Org. Chem. 2019, 84 (8), 4803–4813. https://doi.org/10.1021/acs.joc.8b02811.
(4) Liu, Y.; Navarro-Vázquez, A.; Gil, R. R.; Griesinger, C.; Martin, G. E.; Williamson, R. T. Application of Anisotropic NMR Parameters to the Confirmation of Molecular Structure. Nat. Protoc. 2019, 14 (1), 217–247. https://doi.org/10.1038/s41596-018-0091-9.